| CAS NO: | 444731-52-6 |
| 规格: | ≥98% |
| 包装 | 价格(元) |
| 25mg | 电议 |
| 50mg | 电议 |
| 100mg | 电议 |
| 250mg | 电议 |
| 500mg | 电议 |
| 1g | 电议 |
| Molecular Weight (MW) | 437.52 |
|---|---|
| Formula | C21H23N7O2S |
| CAS No. | 444731-52-6(free base) |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility (In vitro) | DMSO: 87 mg/mL (198.8 mM) |
| Water: <1 mg/mL | |
| Ethanol: <1 mg/mL | |
| Other info | Chemical Name: 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide InChi Key: CUIHSIWYWATEQL-UHFFFAOYSA-N InChi Code: InChI=1S/C21H23N7O2S/c1-13-5-6-15(11-19(13)31(22,29)30)24-21-23-10-9-20(25-21)27(3)16-7-8-17-14(2)28(4)26-18(17)12-16/h5-12H,1-4H3,(H2,22,29,30)(H,23,24,25) SMILES Code: O=S(C1=CC(NC2=NC=CC(N(C3=CC4=NN(C)C(C)=C4C=C3)C)=N2)=CC=C1C)(N)=O |
| Synonyms | GW-78603; GW78603; GW 78603; GW-786034; GW786034; GW 786034; Pazopanib; trade name: Votrient. |
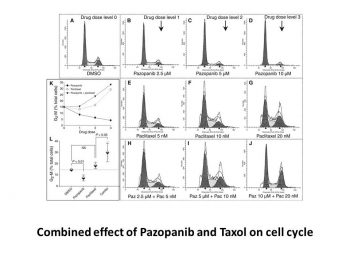
| In Vitro | In vitro activity: Pazopanib potently inhibits VEGF-induced phosphorylation of VEGFR2 in HUVEC cells with IC50 of 8 nM. Pazopanib shows dose-dependent growth inhibition in all synovial sarcoma cell lines including SYO-1 and HS-SY-II cells. Proliferation of SYO-1 and HS-SY-II cells is inhibited even at 1 μg/mL of Pazopanib and is completely abolished at 5 μg/mL. Pazopanib induces G1 arrest, and thereby suppresses the growth of synovial sarcoma cells. Phosphorylation of Akts, GSK-3β, JNKs, p70 S6 Kinase, and mTOR is suppressed in Pazopanib-treated SYO-1 cells compared with that in the vehicle-treated cells. Pazopanib between 20 m g/mL and 22.5 m g/mL shows an increasing reduction of RPE cell viability. Kinase Assay: VEGFR enzyme assays for VEGGR1, VEGFR2, and VEGFR3 are run in homogeneous time-resolved fluorescence (HTRF) format in 384-well microtiter plates using a purified, baculovirus-expressed glutathione-S-transferase (GST) fusion protein encoding the catalytic c-terminus of human VEGFR receptor kinases 1, 2, or 3. Reactions are initiated by the addition of 10 μL of activated VEGFR2 kinase solution [final concentration, 1 nM enzyme in 0.1 M HEPES, pH 7.5, containing 0.1 mg/mL bovine serum albumin (BSA), 300 μM dithiothreitol (DTT)] to 10 μL substrate solution [final concentration, 360 nM peptide, (biotin-aminohexyl-EEEEYFELVAKKKK-NH2), 75 μM ATP, 10 μM MgCl2], and 1 μL of titrated Pazopanib in DMSO. Plates are incubated at room temperature for 60 min, and then the reaction is quenched by the addition of 20 μL of 100 mM ethylene diamine tetraacetic acid (EDTA). After quenching, 20 μL HTRF reagents (final concentration, 15 nM Streptavidin-linked allophycocyanin, 1 nM Europium-labeled antiphosphotyrosine antibody diluted in 0.1 mg/mL BSA, 0.1 M HEPES, pH 7.5) is added and the plates incubated for a minimum of 10 min. The fluorescence at 665 nM is measured with a Wallac Victor plate reader using a time delay of 50 μs. Cell Assay:The IC50 for pazopanib for anchorage-dependent growth was 2 μM and 1 μM after 48 h and 72 h, respectively. Pazopanib abrogated the phosphorylation of VEGFR2 with disruption of downstream PLCγ1. It also disrupted the Ras-Raf-ERK pathway through decreased phosphorylated MEK1/2 and ERK1/2 and affected the phosphorylation of 70S6K. Our findings confirmed that pazopanib targeted endothelial cells, affecting cell growth, VEGFR-induced signaling, and tube formation. |
|---|---|
| In Vivo | Tumor growth in treated mice was significantly delayed (30 mg/kg) or almost totally inhibited (100 mg/kg) compared with the control group. However, tumors rapidly regrew after cessation of treatment at day 30. Using Kaplan–Meier and log-rank analysis, the mean overall survival (OS) was 20 days in the control cohort versus 41 days and 51 days in groups treated with 30 mg/kg and 100 mg/kg pazopanib, respectively. Statistically significant prolongation in mean OS compared with control mice was observed in animals treated with 30 mg/kg and 100 mg/kg. Importantly, treatment with either the vehicle alone or pazopanib did not affect body weight. |
| Animal model | Immune-deficient beige-nude-xid (BNX) mice injected with MM.1S cells |
| Formulation & Dosage | Oral administration, 30 mg/kg and 100 mg/kg |
| References | J Med Chem. 2008 Aug 14;51(15):4632-40; J Orthop Res. 2012 Sep;30(9):1493-8; Retina. 2012 Sep;32(8):1652-63. |
|  |  |
