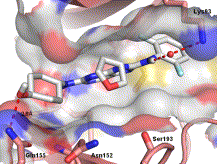
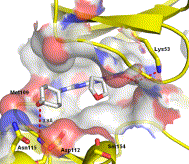
In vitro activity: Tanzisertib (also known as CC-930) is a potent, specific and and orally bioavailable JNK1/JNK2/JNK3 inhibitor that has the potential to be used for the treatment of fibrotic and infammatory indications.Tanzisertib is in Phase 1 clinical trial and may have potential use for prevention and treatment of dermal fibrosis. CC-930 inhibits the formation of phospho-cJun in human PBMC stimulated by phorbol-12-myristate-13-acetate and phytohemeagglutinin (IC50=1 μM). CC-930 (1-2 μM) substantially reduces hepatocyte apoptosis and necrosis, abrogates apoptosis and necrosis in FC-loaded WT hepatocytes. CC-930 blocks the JNK pathway that is activated by pro-fibrotic cytokines in systemic sclerosis.
Kinase Assay: CC-930 is kinetically competitive with ATP in the JNK-dependent phosphorylation of the protein substrate c-Jun and potent against all isoforms of JNK [Ki(JNK1) = 44 ± 3 nM, IC50(JNK1) = 61 nM, Ki(JNK2) = 6.2 ± 0.6 nM, IC50(JNK2) = 5 nM, IC50(JNK3) = 5 nM] and selective against MAP kinases ERK1 and p38a with IC50 of 0.48 and 3.4 μM respectively.
Cell Assay: Systemic sclerosis (SSc) fibroblasts are incubated with 1 μM CC-930 in 96-well plates for 20 h. Then MTT is added at a final concentration of 1 mg/mL, and the cells are further incubated at 37°C for 4 h. Mock-treated fibroblasts are used as controls, and all other results are normalised to untreated cells. |