| CAS NO: | 1028486-01-2 |
| 规格: | ≥98% |
| 包装 | 价格(元) |
| 5mg | 电议 |
| 10mg | 电议 |
| 25mg | 电议 |
| 50mg | 电议 |
| 100mg | 电议 |
| 250mg | 电议 |
| Molecular Weight (MW) | 518.92 |
|---|---|
| Formula | C27H20ClFN4O4 |
| CAS No. | 1028486-01-2 |
| Storage | -20℃ for 3 years in powder form |
| -80℃ for 2 years in solvent | |
| Solubility (In vitro) | DMSO: 27 mg/mL (52.0 mM) |
| Water: <1 mg/mL | |
| Ethanol: <1 mg/mL | |
| Solubility (In vivo) | 15% Captisol: 5 mg/mL |
| Synonyms | MLN-8237; alisertib; MLN8237; MLN 8237 Chemical Name: 4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[c]pyrimido[4,5-e]azepin-2-yl)amino)-2-methoxybenzoic acid SMILES Code: O=C(O)C1=CC=C(NC2=NC=C3C(C4=CC=C(Cl)C=C4C(C5=C(OC)C=CC=C5F)=NC3)=N2)C=C1OC |
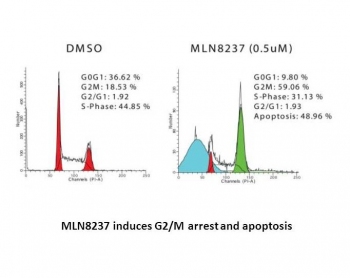
| In Vitro | In vitro activity: MLN8237 shows>200-fold higher selectivity for Aurora A than the structurally related Aurora B with an IC50 of 396.5 nM, and does not have any significant activity against 205 other kinases. MLN8237 (0.5 μM) treatment inhibits the phosphorylation of Aurora A in MM1.S and OPM1 cells, without affecting the Aurora B mediated histone H3 phosphorylation. MLN8237 significantly inhibits cell proliferation in multiple myeloma (MM) cell lines with IC50 values of 0.003-1.71 μM. MLN8237 displays more potent anti-proliferation activity against primary MM cells and MM cell lines in the presence of BM stroma cells, as well as IL-6 and IGF-1 than against MM cells alone. MLN8237 (0.5 μM) induces 2- to 6-fold increase in G2/M phase in primary MM cells and cell lines, as well as significant apoptosis and senescence, involving the up-regulation of p53, p21 and p27, as well as PARP, caspase 3, and caspase 9 cleavage. In addition, MLN8237 shows strong synergistic anti-MM effect with dexamethasone, as well as additive effect with doxorubicin and bortezomib. MLN8237 (0.5 μM) treatment causes the inhibition of colony formation of FLO-1, OE19, and OE33 esophageal adenocarinoma cell lines, and induces a significant increase in the percentage of polyploid cells, and subsequently an increase in the percentage of cells in the sub-G1 phase, which can be further enhanced in combination with cisplatin (2.5 μM), involving the higher induction of TAp73β, PUMA, NOXA, cleaved caspase-3, and cleaved PARP as compared with a single-agent treatment.. Kinase Assay: Aurora A radioactive Flashplate enzyme assay is conducted to determine the nature and degree of MLN8237-mediated inhibition in vitro. Recombinant Aurora A is expressed in Sf9 cells and purified with GST affinity chromatography. The peptide substrate for Aurora A is conjugated with biotin (Biotin-GLRRASLG). Aurora A kinase (5 nM) is assayed in 50 mM Hepes (pH 7.5), 10 mM MgCl2, 5 mM DTT, 0.05% Tween 20, 2 μM peptide substrate, 3.3 μCi/mL [γ-33P]ATP at 2 μM, and increasing concentrations of MLN8237 by using Image FlashPlates. Cell Assay: Cells are exposed to various concentrations of MLN8237 for 24, 48, and 72 hours. Cells viability is measured using MTT assay, and cell proliferation is measured using 3[H]-thymidine incorporation. For cell cycle analysis, cells are permeabilized by 70% ethanol at -20 °C, and incubated with 50 μg/mL PI and 20 units/mL RNase-A. DNA content is analyzed by flow cytometry using BDFACS-Canto II and FlowJo software. For the detection of apoptosis and senescence, cells are stained with fluorescein isothiocyanate-annexin V and PI. Apoptotic cells are determined by flow cytometric analysis using BDFACS-Canto II and FlowJo software. |
|---|---|
| In Vivo | MLN8237 significantly reduces the tumor burden with tumor growth inhibition (TGI) of 42% and 80% at 15 mg/kg and 30 mg/kg, respectively, and prolongs the survival of mice compared with the control. |
| Animal model | Severe combined immune-deficient (SCID) mice inoculated subcutaneously with MM1.S cells |
| Formulation & Dosage | Dissolved in 10% 2-hydroxypropyl-β-cyclodextrin/1% sodium bicarbonate; 30 mg/kg; Oral gavage |
| References | Blood. 2010 Jun 24;115(25):5202-13; Clin Cancer Res. 2011 Dec 15;17(24):7614-24; Mol Cancer Ther. 2012 Mar;11(3):763-74. |
|  |
MLN8237 and CDDP combination treatment exhibits enhanced anti-tumor activity in vivo. Mol Cancer Ther. 2012 Mar; 11(3): 763–774 |
In vitro cell-based phenotypes consistent with Aurora A and Aurora B inhibition at low and high concentrations, respectively. Clin Cancer Res. 2011 Dec 15;17(24):7614-24. |
Pharmacodynamic activity of alisertib in the HCT-116 xenograft model as determined by increased mitotic index, misaligned chromosomes, and reduced bipolar mitotic spindles. Clin Cancer Res. 2011 Dec 15;17(24):7614-24. |
Broad antitumor activity of alisertib in a diverse set of human tumor xenograft models. A, nude mice bearing subcutaneous HCT-116 tumors were dosed with alisertib orally at 3, 10, and 30 mg/kg once daily for 21 consecutive days. Mean tumor volumes (mm3) ± SEM (n = 10 per group) are shown from the beginning of treatment. B, OCI-LY19 tumors inoculated intravenously were treated with alisertib at 20 mg/kg twice daily, 30 mg/kg once daily, and with rituximab at 10 mg/kg once per week. Clin Cancer Res. 2011 Dec 15;17(24):7614-24. |
